The “Right to Repair” is Wrong for Patients
Proper servicing of complex, life-saving and life-sustaining medical devices is vital to their safe and effective functioning and the safety of patients and device users.
From consumer electronics to farm equipment to medical devices, the “right to repair” is in the news as policymakers across the country grapple with what the law should be. The manufacturing and repair of highly complex medical technologies and devices is rigorously regulated by FDA, the global gold standard for medical device safety and efficacy—and for good reason: patient safety is No. 1.
States With Right to Repair Laws
In 2022, New York was the first state to pass comprehensive right to repair legislation. The Digital Fair Repair Act requires original equipment manufacturers (OEMs) to provide documentation, parts, and tools required for diagnosis, maintenance, or repair of digital electronic equipment, and parts for such equipment, to product owners and independent repair providers in New York. The legislature and Governor Kathy Hochul recognized that ensuring patient safety is paramount when it comes to repairing life-saving medical devices and technology and chose to hold the medical device industry harmless from any mandates in the new law. New York’s Digital Fair Repair Act provides a critical precedent going forward as other states consider right-to-repair legislation.
Since the New York law was enacted in 2022, Minnesota (2023), Colorado (2024), and Oregon (2024) have all followed suit and passed comprehensive right to repair legislation for digital electronic equipment. Following New York’s precedent, all three states exempt medical devices from the mandates, ensuring patient safety remains the top priority. As more states introduce legislation on the topic each year, we anticipate the list of comprehensive right to repair laws to grow and will continue to advocate for medical device exemptions in each bill.
Here’s why the “right to repair” complex medical devices is wrong for patients…

Patient safety is paramount.
- Unlike medical device manufacturers, unauthorized third-party servicers are not required to follow FDA regulations or report adverse events.
- The service/repair of a complex device could be a life-or-death matter. OEMs and authorized third-party servicers receive significant training and have extensive expertise for which providing a simple manual is no substitute.
- A 2018 study showed 4,300+ reports of adverse events–including 40 deaths, 294 serious injuries–from devices repaired, replaced, or maintained by third-party servicers.
No credible evidence exists of systemic shortages or delays in equipment repair.
- There is no credible evidence of systemic shortages or delays in equipment repair. Any shortages are intermittent and largely driven by global supply issues that strain access to parts, no matter the servicer.
- Device manufacturers regularly authorize third-party servicers to repair medical devices. There are more than 21,000 companies currently servicing medical devices, according to FDA.

Authorized servicers help save patients time and money.
- Improper servicing can result in increased delays in care.
- In fact, manufacturers are often called in after a third party has failed to repair a machine.
- Some devices require 90+ custom-made tools for servicing.
- Many replacement parts for medical devices are also very specialized and precise in design and function.
- Third-party attempts to duplicate these parts can be disastrous: For some devices, modifications of less than a thousandth of an inch–either through ineffective repair or use of inappropriate replacement parts–can negatively affect device safety, resulting in serious patient injury or death.

Repair mandates are harmful to patients and the innovation they depend on.
- Patients rightly expect medical devices always to perform at a high level and not merely be put back into “working order” by untrained, less-experienced servicers–or worse, not work at all due to substandard methods or parts.
- Improper repair may also increase the risk of exposing patient health data to increased cybersecurity attacks.
- Requiring manufacturers to turn over product keys and manuals effectively eviscerates intellectual property rights and undermines incentives to innovate.

Third-Party Servicing of Medical Devices
Original equipment manufacturers (OEMs) dedicate extensive resources to establishing comprehensive servicing programs to ensure that their devices are properly maintained and continue to meet safety and effectiveness standards as determined by FDA.
OEM servicing programs must follow strict requirements set out in FDA’s Quality System Regulation, which include specific instructions for training, part and component replacement, documentation of servicing activities, and more. In addition, OEMs and their authorized servicers are subject to numerous other FDA regulations covering reporting of adverse events, recalls, registration with the agency, and many others. This comprehensive set of post-market requirements helps ensure that all servicing done to a medical device is done by properly trained, properly equipped technicians; that all work is thoroughly documented; and adverse events are reported to the agency.
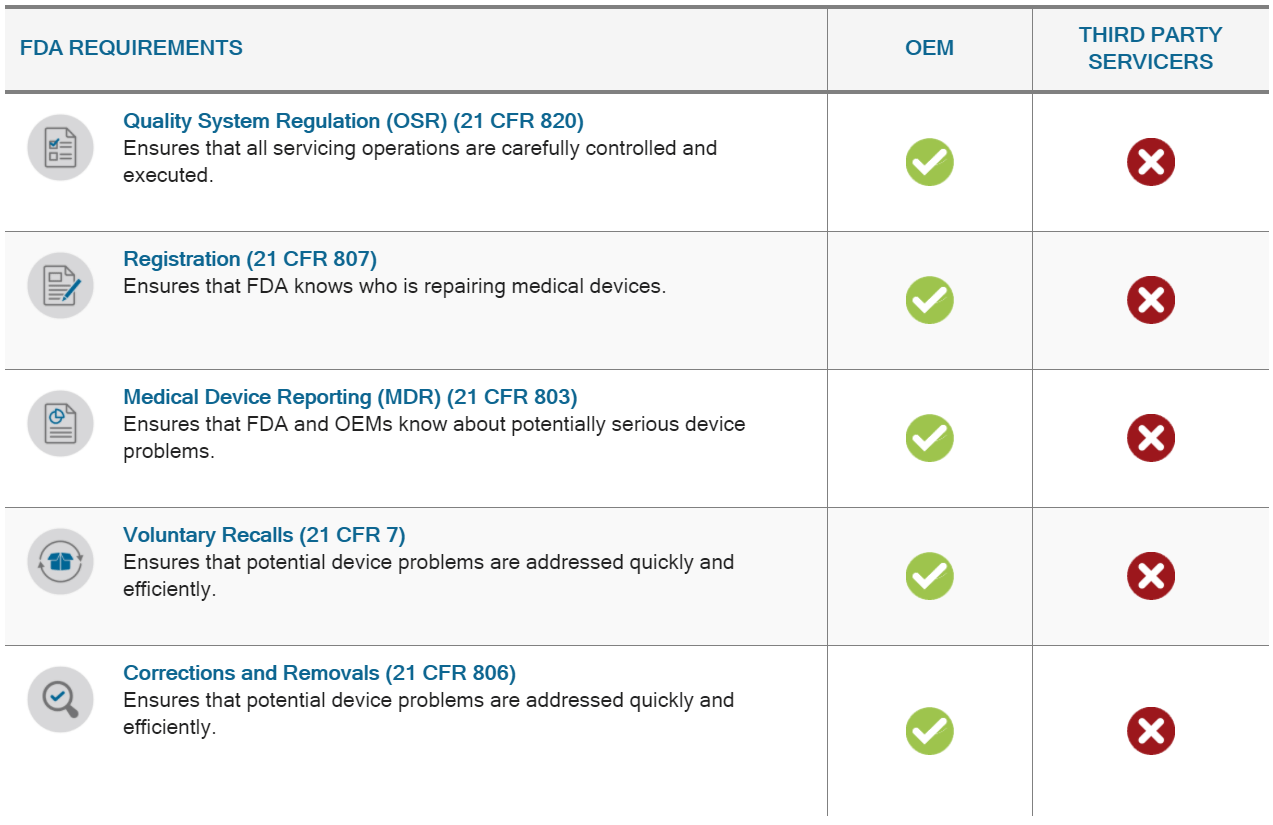
In addition to servicing their own products, many OEMs also act as third-party servicers to other OEMs and comply with FDA regulations. Some OEMs also rely on contracted third-party servicers to meet their own servicing needs and hold these authorized servicers to the same servicing regulations the OEM is required to meet.
However, there are tens of thousands of unregulated third-party servicers working on complex medical devices — ventilators, imaging systems, infusion pumps, patient monitors, etc. — without proper training and sometimes without appropriate equipment and replacement parts. They are not required to follow the same extensive FDA regulatory requirements as OEMs and their authorized servicers, putting patients and device users at risk. They are not even required to submit basic servicing information to FDA, which means the agency and OEMs are essentially flying blind and cannot ensure devices continue to meet their original standard of safety and effectiveness.
Patient Harm from Unregulated Third-Party Servicers
The lack of regulation of third-party servicers has the potential to harm patients. FDA estimated in May 2018 that there were up to 21,000 companies servicing medical devices. The agency further reported that unregulated third-party servicers were associated with more than 4,300 negative incidents involving medical devices, including 40 deaths and 294 serious patient or device user injuries.
To ensure patient safety, third-party servicers should be required to follow the same regulatory requirements as OEMs – including adverse event reporting, registration to enable FDA inspection, and quality system requirements – so the devices they service continue to meet FDA’s high standards of safety and effectiveness.
Fallacy of “Right To Repair”
Proponents of the so-called “Right to Repair” movement demand that unregulated third-party servicers be given unlimited access to service manuals and other proprietary OEM information. Such a move would only serve to put patients and device users at greater risk. Access to the latest manuals is no substitution for the extensive training, knowledge and expertise provided by the OEM.
Even unauthorized third-party servicers that may have previously worked for an OEM and have knowledge or expertise for earlier models of devices on the market are not equipped to continue to work on these devices. The pace of medical technology innovation is so rapid that these technicians’ expertise can quickly become obsolete, rendering their work on newer-model devices potentially dangerous to patients and other device users.
Opinions
Op-Ed by Scott Whitaker & Patrick Hope: ‘Right to Repair’ Push Puts Patients’ Lives at Risk
Op-Ed by G. Wayne Moore: When it comes to advanced medical imaging systems, right to repair is a complex issue
Op-Ed by Dennis Durmis: How the Federal Medical Right to Repair Bill Increases Risk for Patients and Hospitals
Op-Ed by Julianne Malveaux: Why Quality, Regulated Medical Device Repair Is in Patients’ Best Interest
Comments
AdvaMed® Comments on Servicing of Medical Devices Performed by Third-Party Entities
AdvaMed® Comments on Medical Device Servicing and Remanufacturing Activities



