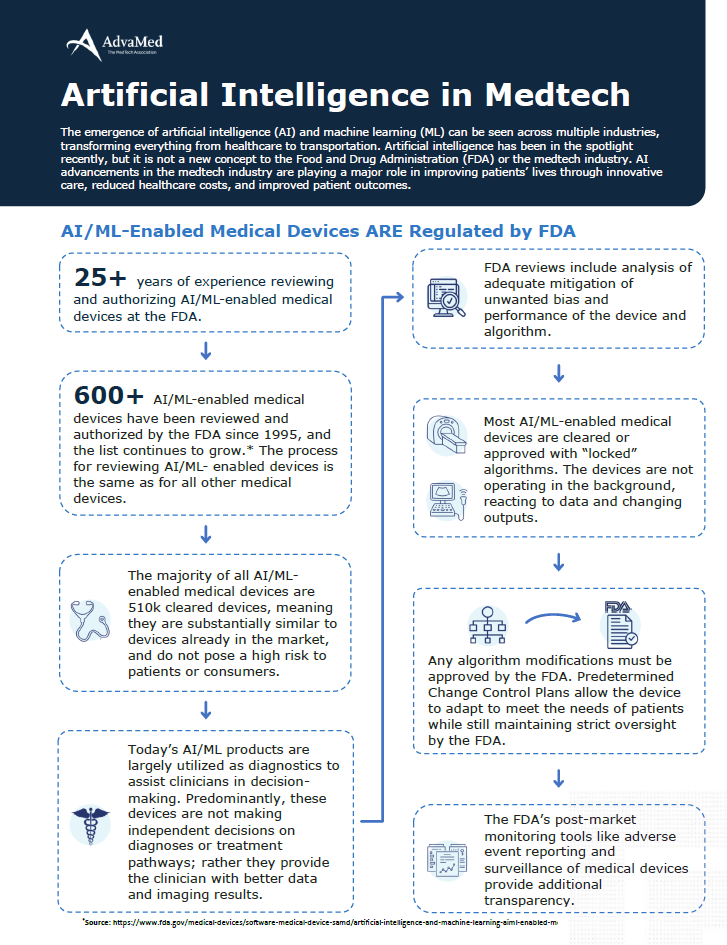
The emergence of artificial intelligence (AI) and machine learning (ML) can be seen across multiple industries, transforming everything from healthcare to transportation. Artificial intelligence has been in the spotlight recently, but it is not a new concept to the Food and Drug Administration (FDA) or the medtech industry. AI advancements in the medtech industry are playing a major role in improving patients’ lives through innovative care, reduced healthcare costs, and improved patient outcomes.
Related Reading
Event / Regulatory Affairs
QMSR Transition: Legal and Audit Perspectives
December 9, 2025
11:00 AM – 12:00 PM
Join Hogan Lovells experts to learn how the FDA is set to reshape the medical device regulatory landscape with the introduction of the QMSR.
Event / Regulatory Affairs
Navigating Regulatory Waters with Medical Devices
December 11, 2025
2:00 PM – 3:00 PM
Get practical guidance from Sedgwick and Morrison Foerster to navigate today’s rapidly evolving medical device regulatory landscape—from recalls to FDA enforcement trends.
News / Digital Health / Regulatory Affairs
AdvaMed Releases Technical Performance and Safety Bulletin on “Remote Device Operations: Common Challenges and Mitigation Strategies”
October 2, 2025
WASHINGTON, D.C. – AdvaMed, the Medtech Association, recently published a technical performance and safety bulletin titled, “Remote Device Operations: Common Challenges and Mitigation Strategies” to help health care providers, device manufacturers, and IT teams navigate the complex landscape of remotely managing medical devices.
News / Government & Legislative Affairs / Regulatory Affairs
CDRH Director Tarver, Fellow FDA Leaders to Speak, Take Questions at The MedTech Conference
September 8, 2025
WASHINGTON, D.C.—AdvaMed, the medtech association, today announced a town hall featuring FDA Center for Devices and Radiological Health (CDRH) Director Michelle Tarver and fellow CDRH senior leaders at The MedTech Conference hosted by AdvaMed in San Diego in October. The town hall participants will update the audience on the center’s strategic direction and priorities and take questions.
Blog / Code of Ethics / Compliance / Legal / Regulatory Affairs / The MedTech Conference
AdvaMed in Action: Legal, Compliance, and Regulatory in the Spotlight at The MedTech Conference 2025
August 27, 2025
Christopher L. White, Esq., AdvaMed’s General Counsel & Chief Policy Officer, on why this year’s legal, compliance & regulatory programming is mission-critical as medtech leaders navigate uncertainty, shifting enforcement priorities, and a rapidly changing global legal landscape.
News / Coverage & Payment / Diagnostics / Digital Health / Government & Legislative Affairs / Health Access / Regulatory Affairs
AdvaMed, Patient Groups, State Medtech Associations Urge Medicare Coverage of Breakthrough Medtech
August 20, 2025
WASHINGTON, D.C.—AdvaMed, the Medtech Association, is among 67 stakeholder groups, including patient advocacy groups and state medtech and life sciences associations, urging the Centers for Medicare and Medicaid Services (CMS) to create a timely, streamlined pathway for Medicare patients to access FDA-authorized breakthrough medical technology.
News / Diagnostics / Digital Health / Government & Legislative Affairs / Medical Imaging / Regulatory Affairs
AdvaMed Welcomes MDUFA VI Discussion Kickoff at FDA Public Meeting
August 4, 2025
WASHINGTON, D.C.—AdvaMed, the medtech association, today welcomed the kickoff of the latest Medical Device User Fee Amendments (MDUFA) discussion at a public meeting at FDA headquarters. Three AdvaMed senior staff members encouraged the agency to build on the successes of the prior five such agreements to ensure the next agreement, MDUFA VI, delivers strong results for the American public.
Event / Digital Health / Regulatory Affairs
AdvaMed® Cybersecurity Summit
November 13, 2025
Join cyber experts for the Cybersecurity Summit that will address the latest industry issues and changes related to FDA requirements.