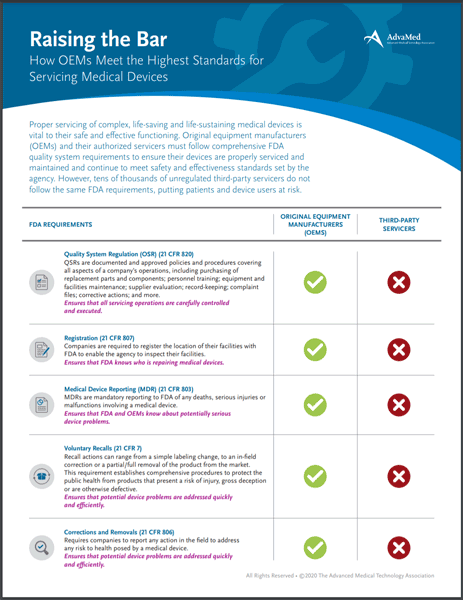
Proper servicing of complex, life-saving and life-sustaining medical devices is vital to their safe and effective functioning. Original equipment manufacturers (OEMs) and their authorized servicers must follow comprehensive FDA quality system requirements to ensure their devices are properly serviced and maintained and continue to meet safety and effectiveness standards set by the agency. This handout examines the differences between OEMs and unregulated third-party servicers who do not follow the same FDA requirements.